Water softeners play a crucial role in enhancing the quality of household water. When hard water, which is high in minerals like calcium and magnesium, flows into your home, it can cause a variety of issues from limescale buildup in pipes to soapy residue on dishes and skin. To tackle these problems, a water softener system employs a method known as ion exchange to replace these hard minerals with sodium ions, effectively transforming hard water into soft water.
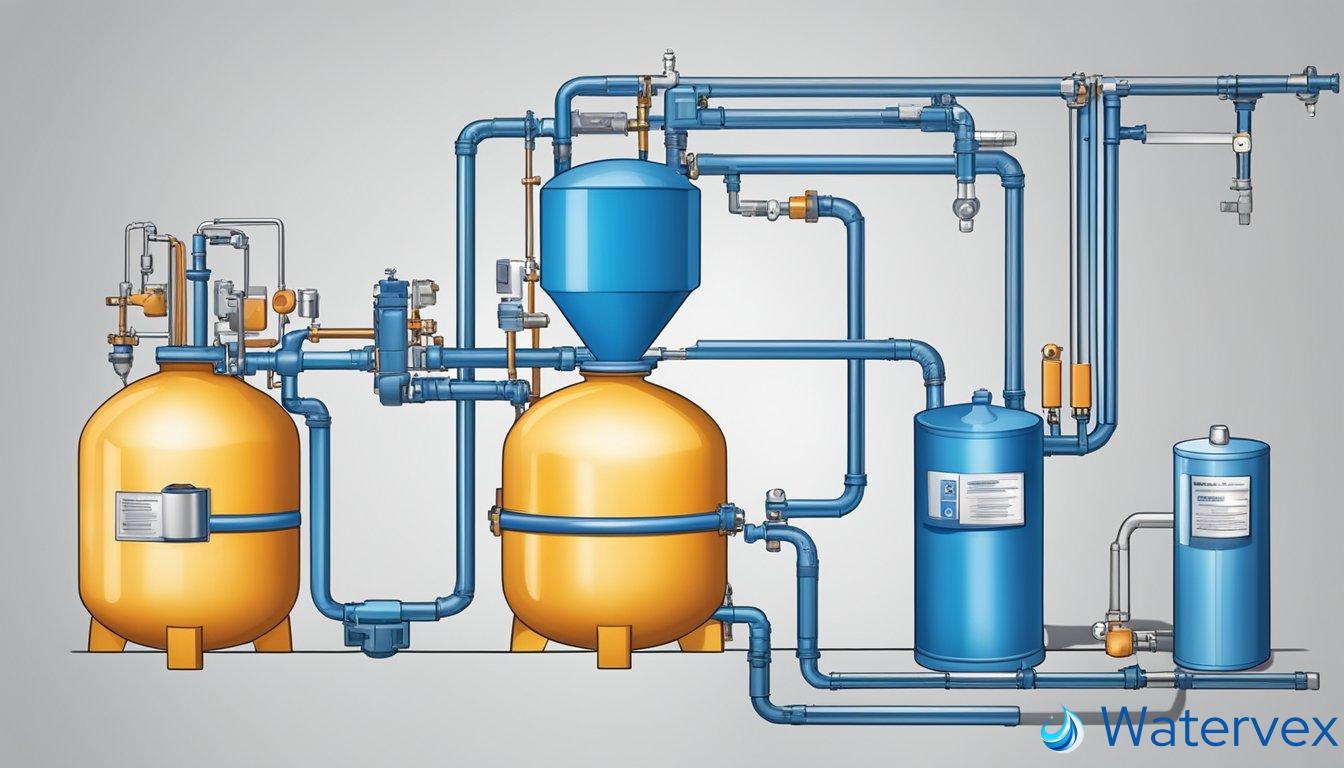
Understanding how a water softener works step by step can help you appreciate the benefits of treated water for drinking, bathing, and cooking, and why it might be a valuable addition to your home. The process begins with water entering the softener’s resin tank, where negatively charged resin beads attract and hold onto the positively charged hard water minerals. During regeneration, these minerals are flushed out of the system, recharging the beads with sodium ions and preparing the unit to soften more water.
Key Takeaways
- Water softeners use ion exchange to remove minerals like calcium and magnesium from hard water.
- Softened water benefits household activities, from cleaning to personal hygiene.
- Regular regeneration of the softener ensures a continuous supply of soft water.
How Water Softeners Function: Step-by-Step Process
To understand the nitty-gritty of how water softeners work, let’s dissect the process and components that play pivotal roles in transforming hard water into soft, usable water for your household needs.
Key Components of the Water Softening Process
Your water softener hinges upon two main parts: the mineral tank and the brine tank. The mineral tank houses resin beads, which are vital to the ion exchange process. Here, calcium and magnesium—the culprits of water hardness—meet their match.
The brine tank assists during the regeneration cycle. It’s filled with a brine solution made from water and sodium ions or potassium chloride. This solution is key to recharging the resin beads with sodium ions after they’ve captured the hard minerals.
Workflow: As hard water enters the mineral tank, it flows through a bed of resin beads.
- These beads are negatively charged and coated with sodium ions.
- Hard minerals like calcium and magnesium, which carry a positive charge, adhere to the beads.
Exchange: This adherence is a result of cation exchange, where resin beads swap their sodium ions for hardness ions.
Cleaning: Over time, beads accumulate minerals and must be renewed, leading to the regeneration cycle.
- The softener’s control valve and meter measure water usage and signal when regeneration is necessary.
- During regeneration, the highly concentrated brine solution flows from the brine tank to the mineral tank.
- The abundance of sodium in the solution forces the minerals off the beads, renewing them for further use.
Effectiveness of Water Softeners in Removing Hardness
Efficiency is an essential aspect; water softeners should effectively eliminate the hardness without frequent regeneration.
- Effectiveness correlates with a perfectly executed regeneration cycle, which determines how well your water softener maintains its softening capability.
- By ensuring the mineral tank is flushed with the correct amount of brine solution, the system can efficiently remove the accumulated hardness and regenerate the resin beads.
By understanding this dual-chamber orchestration and key processes, you can grasp how your system tirelessly works to ensure your water stays soft, and your household runs smoothly.
The Science Behind Ion Exchange and Regeneration
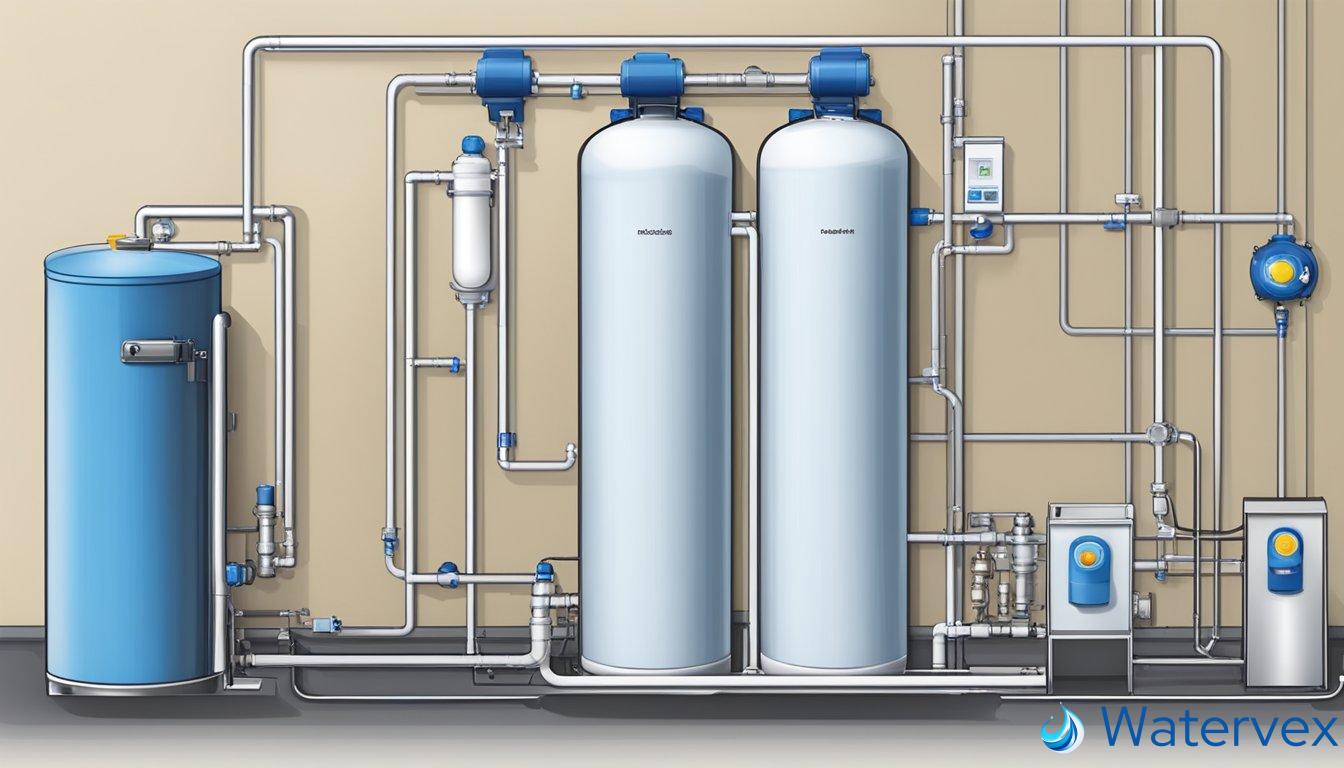
The core of your water softener’s functionality lies in the ion exchange process. Here’s a glimpse into the mechanics: hard water minerals like calcium and magnesium possess a positive charge. These ions are no match for the negatively charged sites within the resin tank of your softener, leading to their capture and replacement by sodium or potassium ions.
Let’s break it down:
- Hard Water Entry: Hard water flows into the resin tank filled with polystyrene beads, which are negatively charged.
- Ion Swap: As these minerals cling to the beads, sodium or potassium ions, which are less tenacious, are released into your water in their place.
The clever bit comes with regeneration. This step is vital to maintain the softener’s efficacy. Over time, the capture of hard minerals saturates the resin beads, hindering their performance. This is where salt pellets and a brine solution come into play.
Regeneration involves:
- The brine solution, rich in sodium, floods the resin tank during the backwash cycle.
- The high concentration of sodium compels the depleted beads to release the captured hard minerals and grab onto new sodium ions.
- The freed minerals, now in the brine, are flushed out of the system.
This restores the softener to its full softening glory, ready to tackle more hard water minerals. Remember, the choice of salt or potassium affects the regeneration, influencing the rates at which these exchanges and flushes occur.
As you engage in this maintenance, bear in mind the direct impact on the efficiency and longevity of your plumbing and appliances, not to mention the difference in water quality you’ll notice.
Health and Practical Benefits of Soft Water
When it comes to soft water, your health and home reap benefits. Soft water, unlike its hard counterpart, lacks high mineral concentrations, which makes it kinder to your skin and hair. You might notice your skin feels softer and less dry after switching to soft water because it doesn’t strip away natural oils like hard water can.
Limescale, a chalky deposit caused by minerals in hard water, is greatly reduced with a water softener. By mitigating mineral buildup, soft water ensures your faucets and appliances run more efficiently. Less limescale means a longer life for your washing machine and less energy required to heat your water, which could translate into savings on your utility bills.
Less mineral buildup also means less time and effort spent cleaning. Soft water combats soap scum and mineral deposits, making it easier to maintain a clean and inviting home. Soap lathers more effectively in soft water, resulting in its more efficient usage and further financial savings.
The absence of excess minerals like calcium, magnesium, and manganese in your water can be a boon for those manufacturing or using products that require water. A water softener ensures that the water you use is consistent and of predictable quality, which is essential for the operation of many home appliances as reflected by the advice of various appliance manufacturers.
Remember, all licensed products, including water softeners, should be researched and installed according to the manufacturer’s specifications to ensure they perform effectively in reducing unwanted mineral content in your home’s water supply.
Comparing Water Softeners to Alternative Solutions
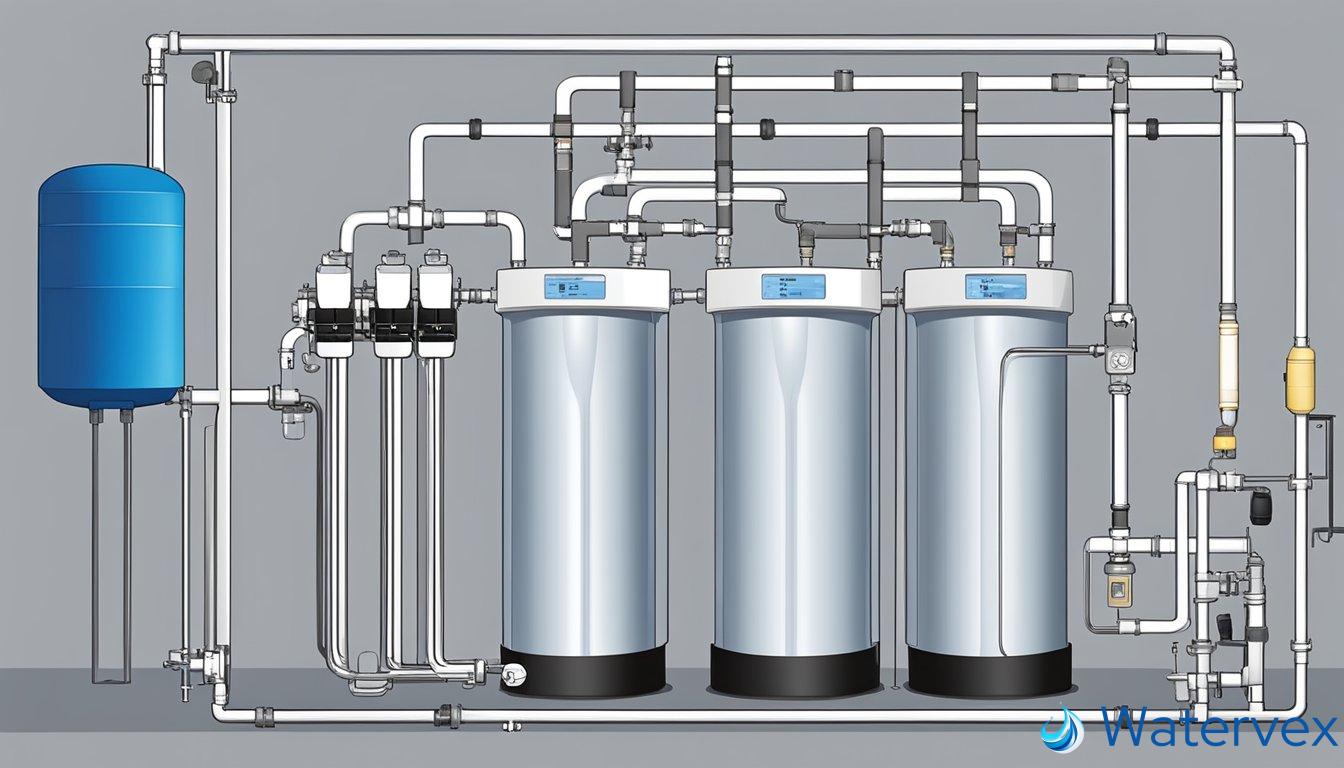
When investigating solutions for enhancing the quality of your home’s water, you might be weighing traditional water softeners against other technologies like salt-free systems or reverse osmosis. Your choice will significantly affect how dissolved minerals are managed in your municipal or well water.
Water Softeners
Traditional water softeners replace calcium and magnesium ions with sodium or potassium ions, a process essential for preventing scale buildup. Though effective, keep in mind you’ll need to periodically replenish the bags of salt.
- Pros: Comprehensive water softening system.
- Cons: Requires ongoing salt purchases and maintenance.
Salt-Free Systems
A salt-free system, such as a water conditioner, neutralizes minerals rather than removing them, reducing scale without adding sodium. This might be important if you’re concerned about salt intake.
- Pros: Maintenance-free with no salt needed.
- Cons: Minerals remain, potentially affecting taste and not technically softening.
Reverse Osmosis
Reverse osmosis filters out impurities, including minerals, at a meticulous level using a membrane. Ideal if you’re seeking pure water for both drinking and household use.
- Pros: Filters out a vast array of contaminants.
- Cons: More costly, wastes water, and removes beneficial minerals.
Outerlying factors like your household size, water use, and local water quality can sway your decision. Remember, while water conditioning may seem to offer a one-size-fits-all solution, it doesn’t truly soften water, but it does prevent minerals from sticking to surfaces. Meanwhile, reverse osmosis systems demand a higher investment and come with increased water waste. Choose based on what aligns with your family’s needs and the specifics of your water supply.

